Abstract
Introduction: Clonal hematopoiesis (CH) involves the accumulation of clonal hematopoietic cells in the context of largely normal peripheral blood counts. While somatic mutations in these clones (CH of indeterminate potential = CHIP) are associated with myeloid malignancies and increased cardiovascular risk in patients (pts), mosaic copy number alterations (mCA) can also drive CHIP and associated risk (Niroula, Nat Med 2021). Pts with relapsed/refractory (r/r) lymphomas carrying CHIP mutations prior to autologous transplantation have inferior outcomes (Gibson, JCO 2017). Given the high frequency of PPM1D mutations identified in these groups, a significant share of these mutations is likely therapy-associated. The role of CHIP and mCA in newly diagnosed lymphomas remains poorly described.
Methods: We studied 153 pts with previously untreated diffuse large B-cell lymphomas (DLBCL), and 60 with r/r diesease. Newly diagnosed DLBCL were treated within the Phase III PETAL trial with R-CHOP or a Phase II with Lenalidomide and Obinutuzumab + CHOP. The second cohort of 60 pts with r/r disease was included who underwent Axicabtagene Ciloleucel (Axi-cel) therapy at Stanford. Non-silent mutations in 17 canonical CHIP genes at variant allele frequencies ≥2% were genotyped using Cancer Personalized Profiling by Deep Sequencing (CAPP-Seq) of leukocytes isolated from the peripheral blood. mCA were called from the same leukocyte specimens by statistical integration using CANARy (Chabon et al 2020 Nature).
Results: CHIP mutations were found in 17/153 pts (11%) with treatment-naive DLBCL and 9 of 60 pts (15%) with r/r disease. The gene most affected by CHIP in untreated pts was DNMT3A (65%) followed by TET2 (18%). In contrast, in pts with r/r disease, genes involved in DNA repair PPM1D (33%) and TP53 (22%) were most frequently mutated. mCA were detected in 5 patients (3%) with untreated DLBCL, with only one patient had an alteration in a previously described region associated with myeloid mCA (Niroula, Nat Med 2021). CHIP was associated with age in newly diagnosed DLBCL (p <0.01), but not IPI Score, tumor volume, or circulating tumor DNA (ctDNA) levels in plasma.
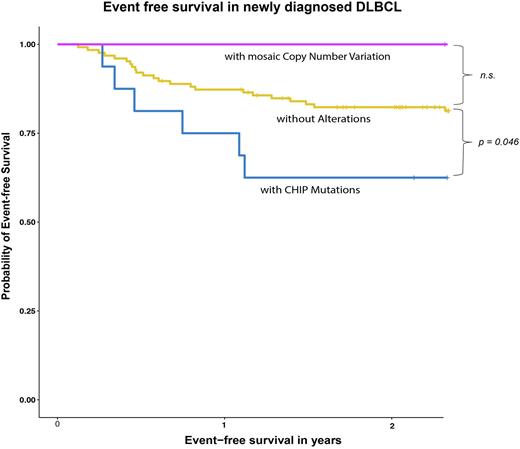
Strikingly, previously untreated pts with CHIP had a significantly reduced event-free survival (EFS) than pts without CH (p = 0.046) (Figure 1) following immunochemotherapy with curative intent. In contrast, no such impact of CHIP was observed in r/r DLBCL treated with Axi-cel (p = 0.99) or in patients with mCA (p = 0.3). CHIP was associated with lower DLBCL response rates: among evaluable pts without CHIP, 98% achieved a complete or partial remission (CR/PR) compared to only 88% in pts with CHIP (p = 0.1). Of note, therapy-related myelosuppression appeared similar in newly diagnosed DLBCL pts with and without CHIP, as we observed no significant difference in delivered dose intensity, transfusion needs, or infection rates.
Conclusions: Clonal hematopoiesis is pervasive at diagnosis of DLBCL involving both somatic mutations and copy number lesions. While somatic mutations driving CHIP are associated with lower response rates and inferior survival outcomes after frontline chemoimmunotherapy of DLBCL, this effect was not observed for axi-cel therapy of r/rDLBCL pts. Of interest, the mutational CHIP landscape differed between the the pre-treatment and r/r cohorts, suggesting that cytotoxic therapy shapes CHIP evolution.
Disclosures
Alig:Takeda Pharmaceuticals: Consultancy. Westin:Genentech/Roche: Consultancy, Research Funding; MonteRosa: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Merck: Consultancy; Calithera: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Iksuda: Consultancy; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Abbvie/GenMab: Consultancy; SeaGen: Consultancy. Miklos:Novartis: Consultancy; Bristol Meyers Squibb: Consultancy; Allogene: Research Funding; Pharmacyclics: Patents & Royalties: cGVHD Ibrutinib patent ; Janssen: Consultancy, Honoraria; Kite, a Gilead Company: Research Funding; Adaptive Biotech: Consultancy; Fosun Kite: Consultancy, Honoraria. Frank:Adaptive Biotechnologies: Consultancy, Honoraria, Research Funding; Allogene Therapeutics: Research Funding; Kite/Gilead: Honoraria, Research Funding; Roche/Genentech - Wife: Current equity holder in private company, Current holder of stock options in a privately-held company. Diehn:Foresight Diagnostics: Consultancy, Current equity holder in private company. Kurtz:Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; Genentech: Consultancy; Roche: Consultancy; Adaptive Biotechnologies: Consultancy. Alizadeh:Karyopharm: Consultancy; Roche: Consultancy; Syncopation: Current equity holder in private company, Patents & Royalties; Cibermed Inc: Consultancy, Current equity holder in private company, Patents & Royalties; Gilead: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties; Adaptive Biotechnologies: Consultancy; Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; Genentech: Consultancy; BMS: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.